Public swimming pools are chlorinated to reduce bacterial and algal contamination. There are several ways to
add chlorine, which should preferably have a concentration of 2–3 ppm (minimum concentration 1 ppm). The pH of the water is
then adjusted to about 7.5 by the addition of acid or alkali. Sources of chlorine are sodium hypochlorite, which has an alkaline
pH and, thus, no potential to cause erosion in teeth; chlorine gas, which is used mainly in large public swimming pools; and “stabilized” chlorine, which is created by combining chlorine and the salts of cyanuric acid into a tablet form, usually about the size of a hockey
puck. In solution, chlorine generates hypochlorous and hydrochloric acids, the former having disinfectant properties. Cyanuric acid retards the rate at which hypochlorous acid is broken down by sunlight. Unless the acids are neutralized, usually with sodium carbonate,
the pH of the water may be less than 3. A low pH may not be sensed by swimmers, although it may cause eye irritation in those
not wearing goggles; however, acidic water in contact with the teeth will cause irreversible erosion of the enamel.
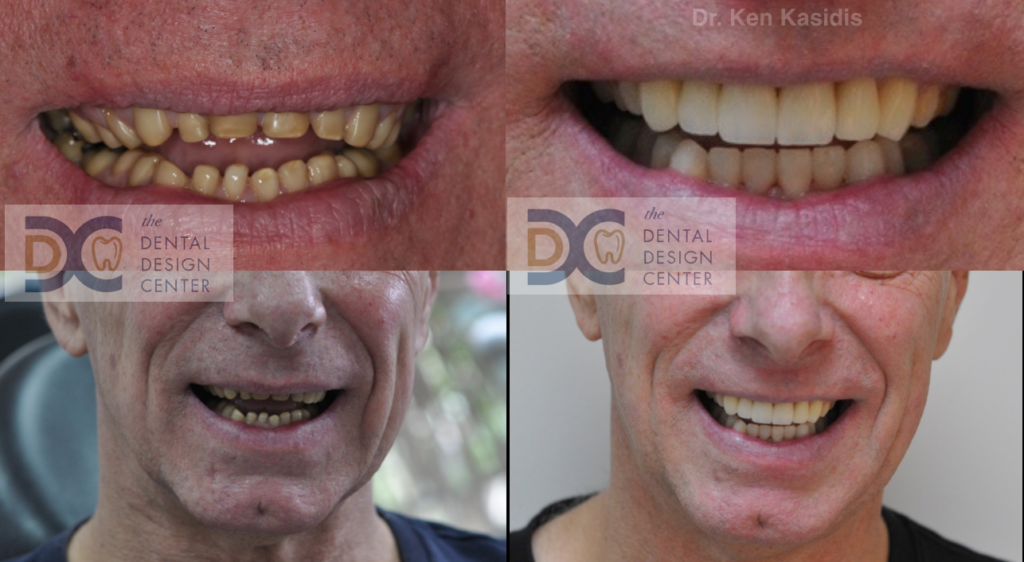
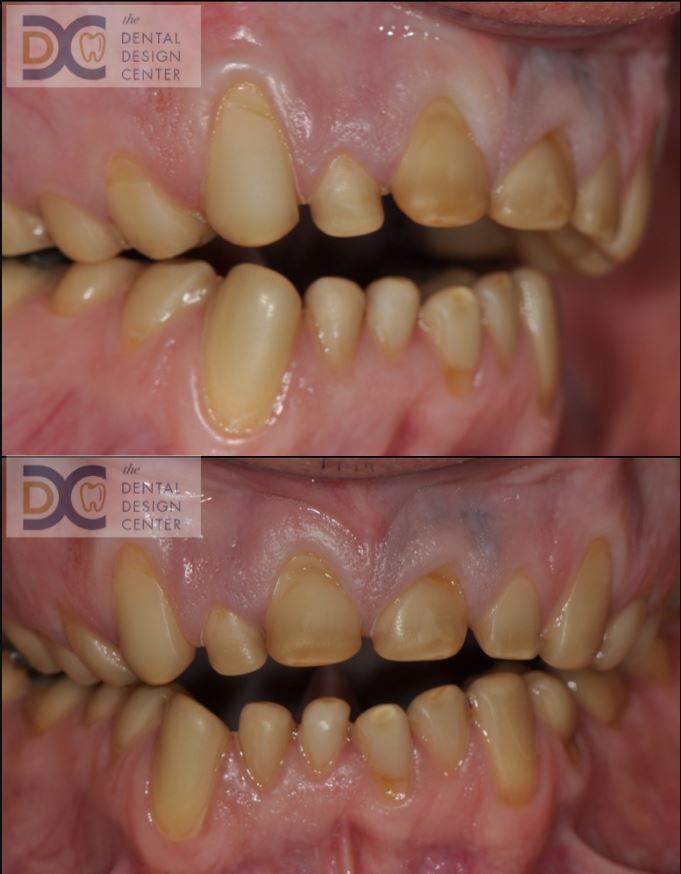
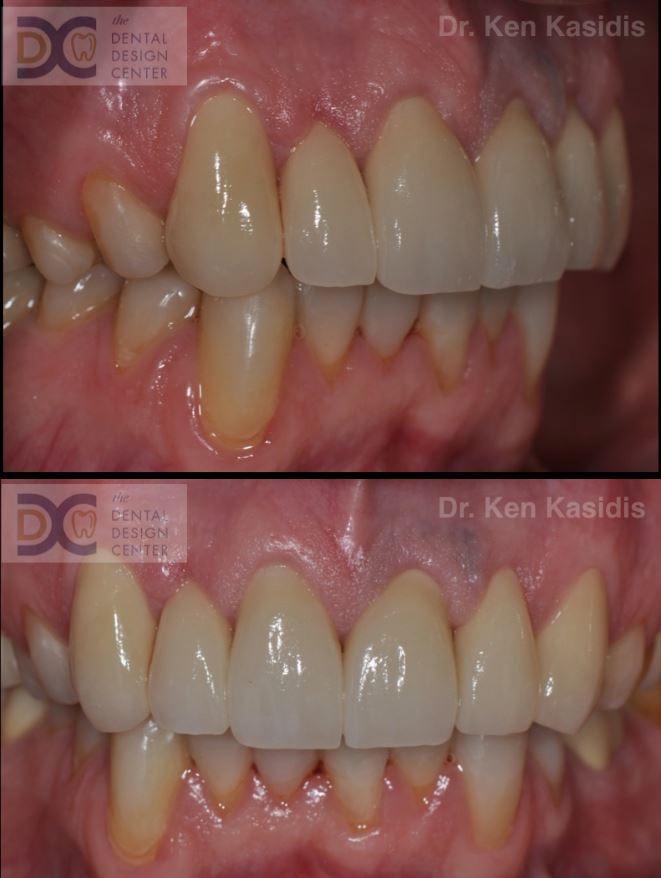
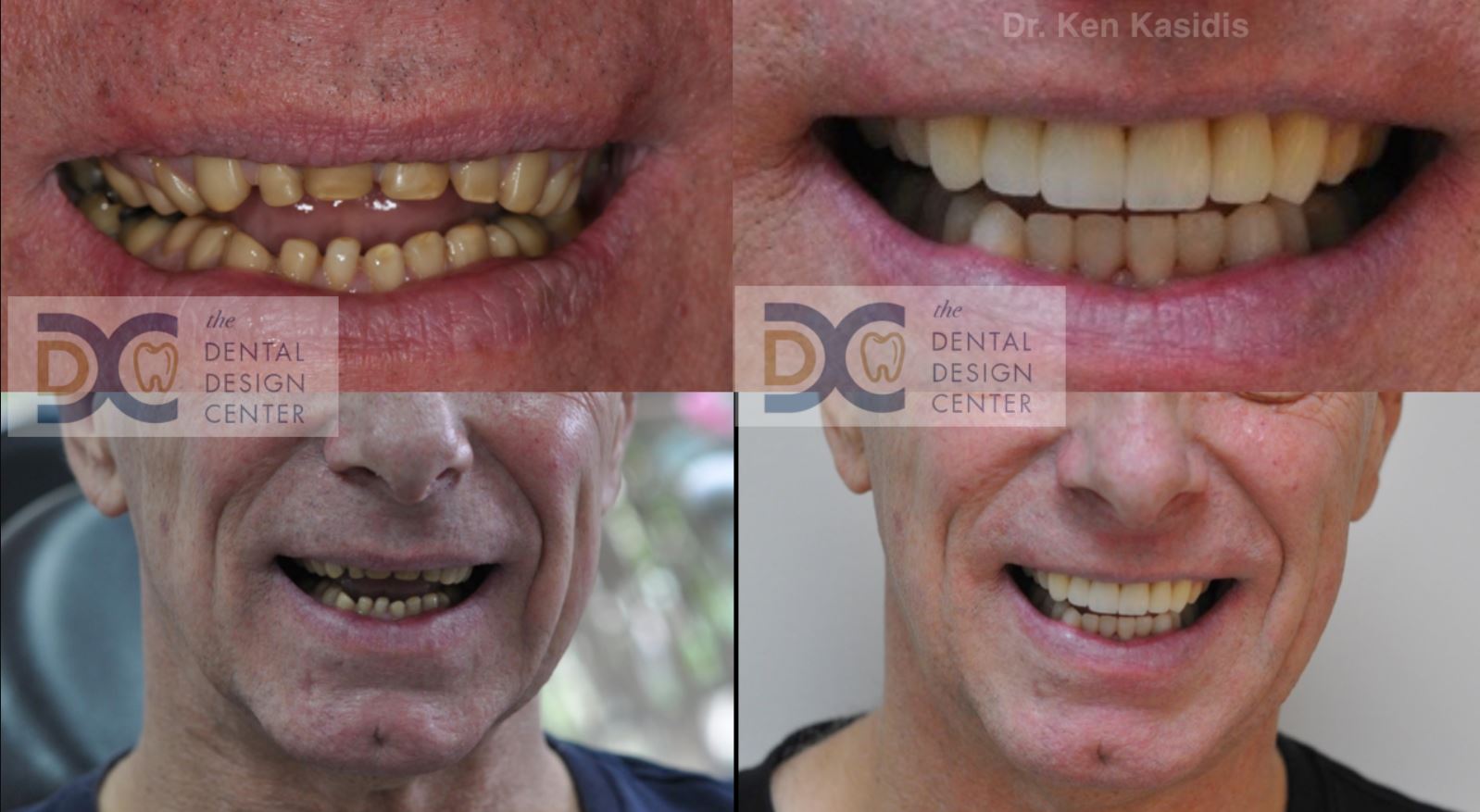
Pictures show severe erosion of tooth enamel of the patient who has history of swimming in the chlorinated swimming pool every day for nearly 20 years. At the time that he visited us, he just noticed that his teeth were wearing down so quickly and there were diastemas between his front teeth.

Reference:
Dawes C and Boroditsky CL. Rapid and Severe Tooth Erosion from Swimming
in an Improperly Chlorinated Pool: Case Report. May 2008, Vol. 74, No. 4: 359-361